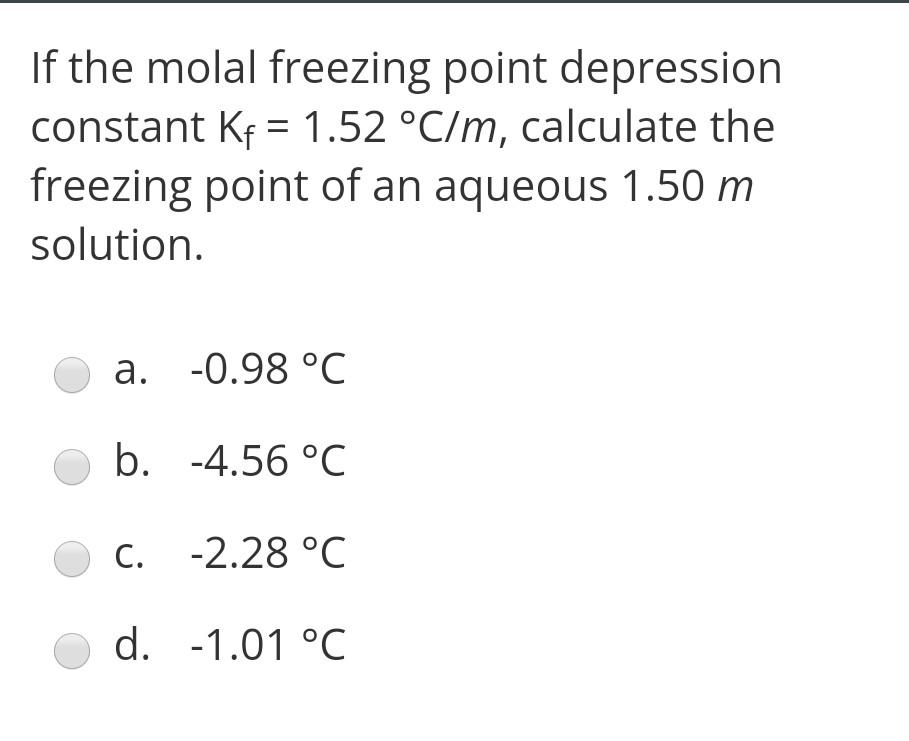
Calculate Complex Ion Equilibria Using the Small x Approximation for Large Kf | Chemistry | Study.com

After you preform your experiment, you determine that the Kf value for naphthalene is 6.9 . You are using 10g of naphthalene and added 1.0 g of your unknown. The the freezing

Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (kf for water = 1.86 K kg mol^-1)

SOLVED:From the value of Kf listed in Table 17.1, calculate the concentration of Ni^2+ in 1.0 L of a solution that contains a total of 1 ×10^-3 mol of nickel(II) ion and
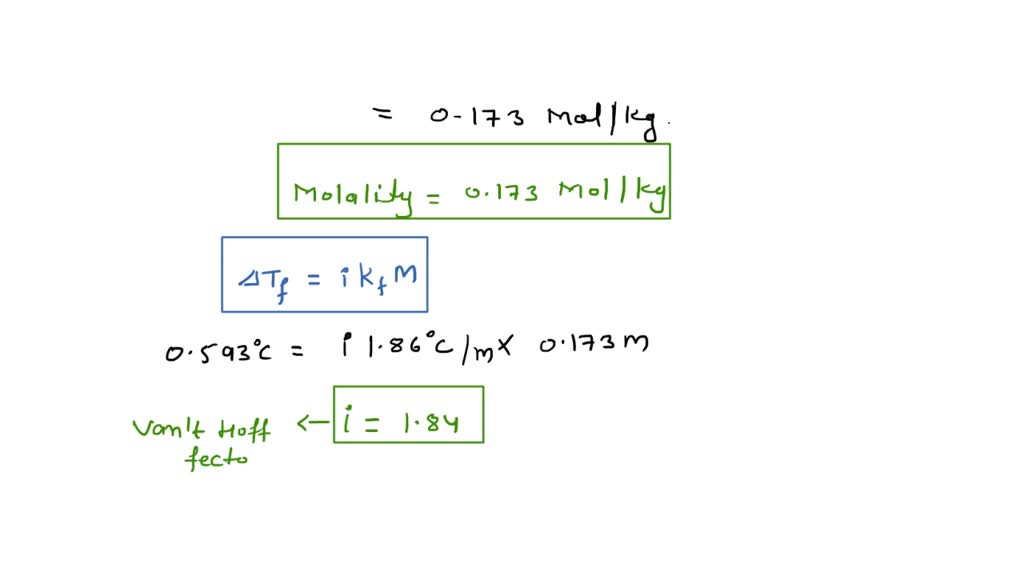
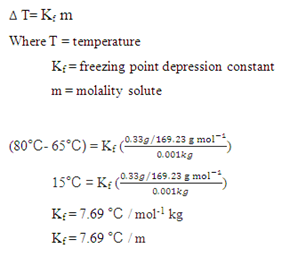
SOLVED: 1) Determine the freezing point for a solution with a concentration of 0.100 m K2SO4 in acetic acid (Tf = 16.6 oC, Kf = -3.90 oC/m). a.) Calculate the freezing point

How are Kf values relevant in calculations of the melting temperature of a solution? | Homework.Study.com