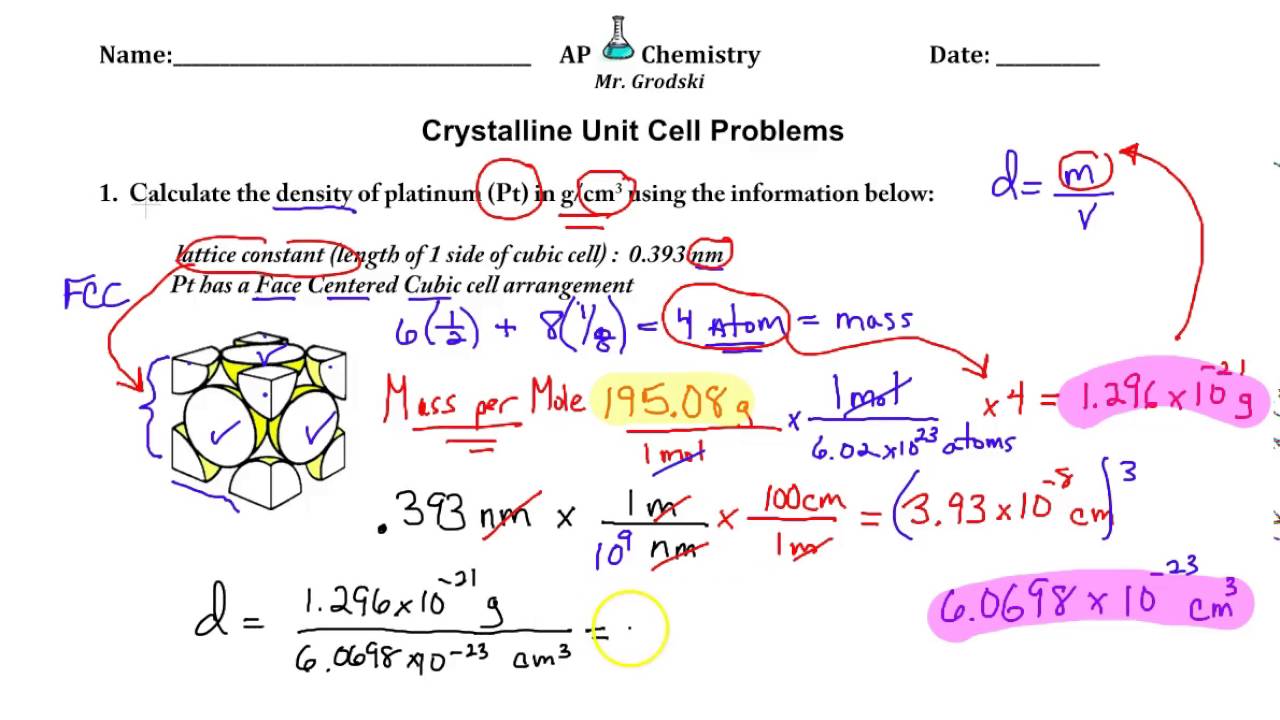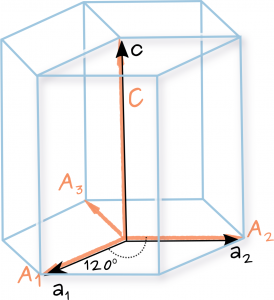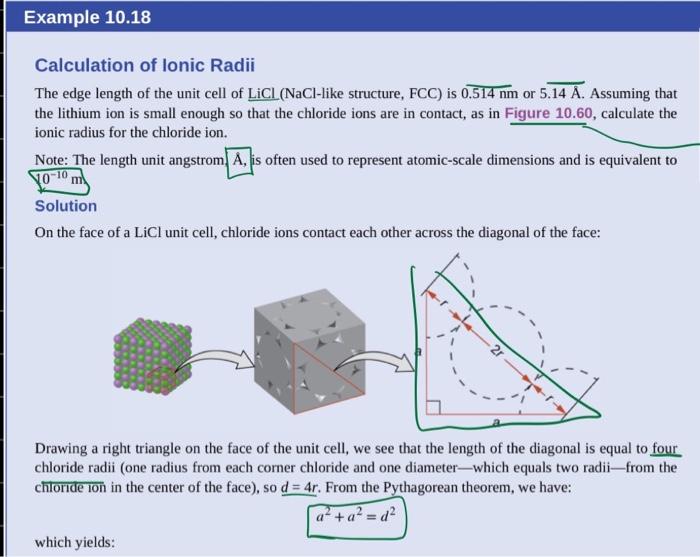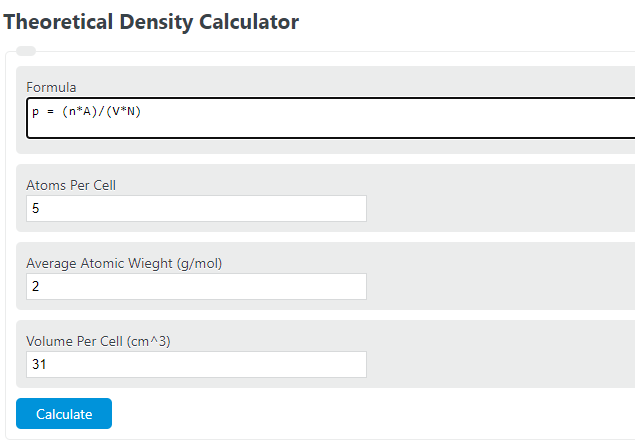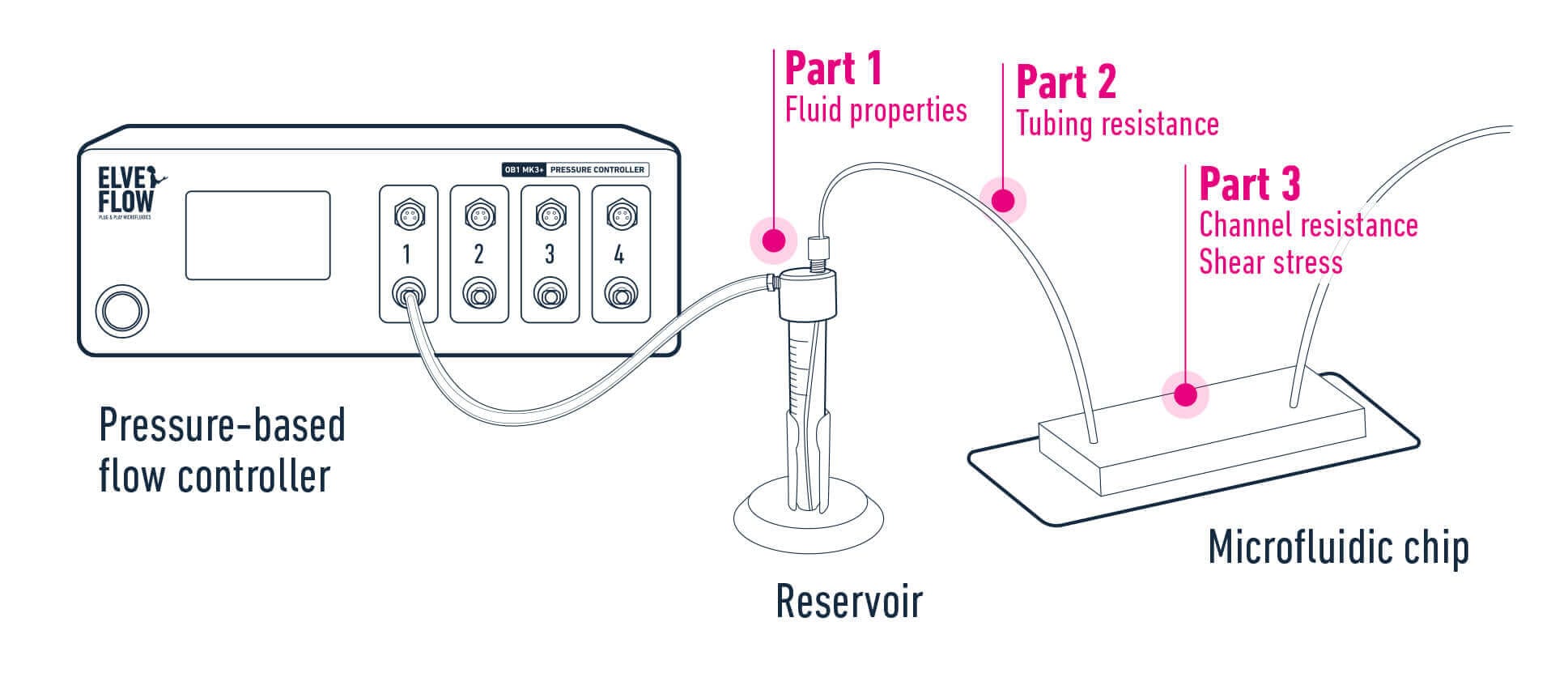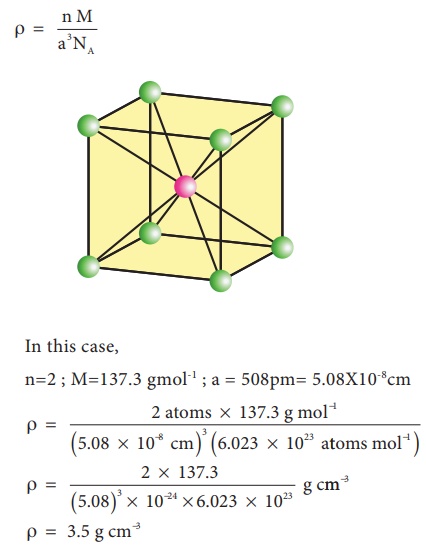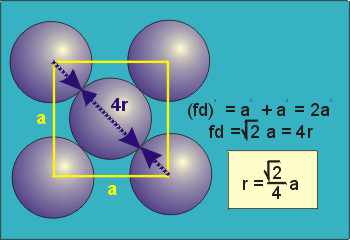
A certain element exists in a face-centered cubic structure. The atomic radius of an atom of this element is 185 pm. What is the density of this element in g/cm3?(Assume the element
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering

CT-9240C-W New Arrival 14 Large Display Dual Power Calculator with 1 PC AA Cell workable Unit Size 20*15.5*4cm Hotsale Wholesale in Africa_Calculator - winningmart.com

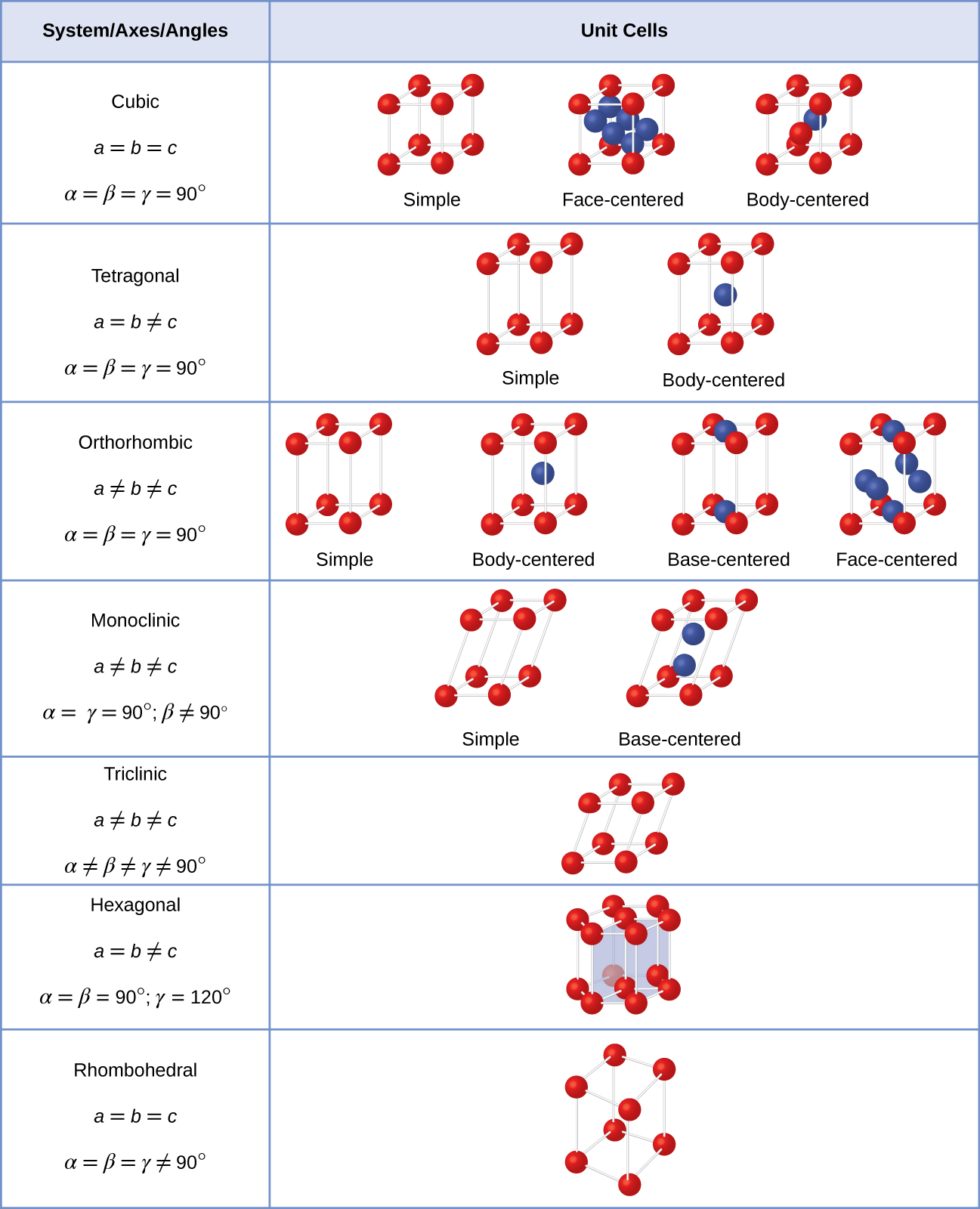
What is a Unit Cell? - Definition, Types of Unit Cell, Primitive Unit Cell, BCC & FCC, Volume of HCP Unit Cell

What is the formula to calculating the lattice parameter or lattice constant of Orthorhombic structure? | ResearchGate